马上注册,结交更多好友,享用更多功能,让你轻松玩转社区。
您需要 登录 才可以下载或查看,没有账号?立即注册

x
有人说21位特优于易,但据日本人最新的研究报道,没区别。以下是英文摘要:
Randomized Phase III Study Comparing Gefitinib With
Erlotinib in Patients With Previously Treated Advanced Lung
Adenocarcinoma: WJOG 5108L
Purpose
The epidermal growth factor receptor (EGFR) tyrosine kinase has been an important target for
non–small-cell lung cancer. Several EGFR tyrosine kinase inhibitors (TKIs) are currently approved,
and both gefitinib and erlotinib are the most well-known first-generation EGFR-TKIs. This
randomized phase III study was conducted to investigate the difference between these two EGFRTKIs.
Patients and Methods
Previously treated patients with lung adenocarcinoma were randomly assigned to receive gefitinib
or erlotinib. This study aimed to investigate the noninferiority of gefitinib compared with erlotinib.
The primary end point was progression-free survival (PFS).
Results
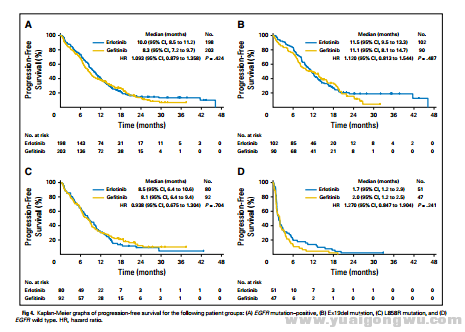
Five hundred sixty-one patients were randomly assigned, including 401 patients (71.7%) with EGFR
mutation. All baseline factors (except performance status) were balanced between the arms.
Median PFS and overall survival times for gefitinib and erlotinib were 6.5 and 7.5 months (hazard
ratio [HR], 1.125; 95% CI, 0.940 to 1.347; P = .257) and 22.8 and 24.5 months (HR, 1.038; 95% CI,
0.833 to 1.294; P = .768), respectively. The response rates for gefitinib and erlotinib were 45.9% and
44.1%, respectively. Median PFS times in EGFR mutation–positive patients receiving gefitinib
versus erlotinib were 8.3 and 10.0 months, respectively (HR, 1.093; 95% CI, 0.879 to 1.358;
P = .424). The primary grade 3 or 4 toxicities were rash (2.2% for gefitinib v 18.1% for erlotinib) and
alanine aminotransferase (ALT)/aspartate aminotransferase (AST) elevation (6.1%/13.0% for gefitinib v 2.2%/3.3% for erlotinib).
Conclusion
The study did not demonstrate noninferiority of gefitinib compared with erlotinib in terms of PFS in
patients with lung adenocarcinoma according to the predefined criteria.
J Clin Oncol 34. © 2016 by American Society of Clinical Oncology |
-
|
|
|
|
|
|
共1条精彩回复,最后回复于 2016-9-15 19:11

尚未签到