本帖最后由 老马 于 2012-1-18 09:42 编辑 2 r/ q9 f; o1 F! ]; b
4 ~* ^! Y; T% v2 C j( \阿瓦斯丁治疗肺鳞癌的安全性
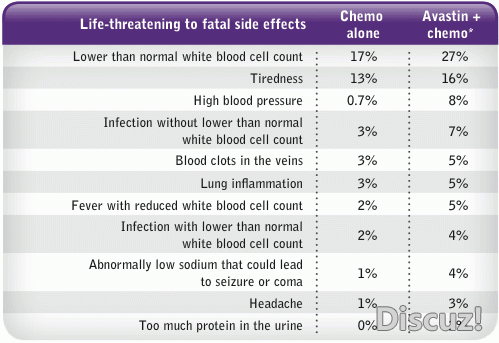
" X9 @0 h$ |8 M贝伐单抗最严重的不良反应是肿瘤相关性出血,与鳞状细胞癌、肿瘤坏死、空洞形成及肿瘤靠近大血管有关,并可引起高血压等心向管不息反应。
1 L, T) U1 }1 [2 e, w
 7 g0 s z6 L* R2 i8 S
7 g0 s z6 L* R2 i8 S
BRIDGE: An open-label phase II trial evaluating the safety of bevacizumab (BV) plus paclitaxel/carboplatin (PC) as first-line treatment (tx) for patients (pts) with advanced, previously untreated, squamous non-small cell lung cancer (NSCLC).$ ]/ z; { f+ i/ b$ d, Q
Background: In a phase II study of first-line tx with BV + PC for NSCLC, squamous histology was found to be a risk factor for severe (Gr ≥ 3) pulmonary hemorrhage (PH). Subsequently, pts with predominantly squamous histology were generally excluded from phase III studies. This final analysis from the BRIDGE study assesses safety of delayed BV administration in pts with locally advanced or metastatic squamous NSCLC. Methods: BRIDGE was an open-label, single-arm, multicenter pilot study of PC alone in cycles 1 and 2, and PC + BV in cycles 3 to 6, followed by BV until progressive disease (PD) or unacceptable toxicity. Eligible pts had stage IIIb (with pleural effusion), stage IV, or recurrent squamous NSCLC. Pts with recent arterial thromboembolic events, gastrointestinal perforation, hemoptysis, untreated brain metastases, intrathoracic lesion(s) with cavitation, or anticoagulation therapy were not eligible. The primary endpoint was incidence of Gr ≥ 3 PH. Results: Forty- seven pts received 2 initial cycles of PC, and 31 continued on study to receive ≥ 1 dose of BV. Median age was 65 (range: 48-80); 96.3% had Stage IV disease. Pts received a median of 6.0 (range, 1-18) doses of BV, and 2 pts (6.5%) completed 12 mos of tx. Among the 31 pts who received BV, there were 3 reports of PH of any grade in 2 pts. One pt had Gr 1 PH during cycle 3, withdrew due to PD on the same day, and recovered from PH. A second pt developed Gr 3 PH after post-progression treatment, 44 days after the last BV dose; PH resolved 2 d later, but after another 55 d the pt developed Gr 4 PH, and subsequently died due to PD. Incidence of Gr ≥ 3 PH (1 pt) was 3.2% (90% CI: 0.3-13.5%). Nine pts (29.0%) experienced Gr 3 events, including 5 (16.1%) with hypertension; 5 experienced Gr 4 events (dyspnea, PH, basal ganglia infarction, cerebral ischemia and pain). Median progression-free survival (PFS) was 6.2 mos (95% CI: 5.32-7.62 mos); 57% of pts had PFS of ≥ 6 mos. Conclusions: The incidence of Gr ≥ 3 PH in this study was 3.2% (1 pt). No new safety signals were identified. Until further trials are conducted, tx of squamous NSCLC with BV should be considered experimental., v+ T$ q% E" Q V; o0 U
 9 [; q+ ^! W2 z% s
9 [; q+ ^! W2 z% s
 Microsoft Word - 阿瓦斯丁™_AVASTIN_中文说明书.pdf
(242.23 KB, 下载次数: 527)
Microsoft Word - 阿瓦斯丁™_AVASTIN_中文说明书.pdf
(242.23 KB, 下载次数: 527)
7 G- `4 U7 Z( m. N S( Y" z9 q |